I had reported earlier that once perfected and approved by regulators, safe and robust ambulatory artificial pancreas ‒ or to use the scientific term ‘closed loop insulin delivery system’ ‒ has the potential to greatly improve the health and lives of people with type 1 diabetes. The idea itself is not new but the old generation closed loop insulin delivery systems were cumbersome and unsuitable for long term or outpatient use.
Moreover, the control algorithm used by Hovorka and colleagues belongs to an advanced class of closed loop control technologies known as “model predictive control”. Algorithm designs for artificial pancreas have generally used either “proportional-integral-derivative control” or “model predictive control”.
Proportional-integral-derivative control algorithms are reactive, responding to changes in glucose levels with adjustment in insulin delivery. Model predictive control algorithms are built over a model of the human metabolic system and are therefore proactive, delivering insulin in anticipation of changes in glucose concentrations.
This compensates partially for the time delays inherent in subcutaneous glucose control (the time delay in insulin action, which can amount to 60 minutes or more). For this reason, model predictive control has become the approach of choice more recently.
The algorithm developed by Hovorka and colleagues has certain distinct features, such as real time adaptation of the underlying model to changing patient parameters implemented as a selection from several predefined models. However, this potential advantage remains to be evaluated.
Most importantly, this is one of the first studies to test realistic meal scenarios and challenge the participants with a large dinner that included alcohol. As such, the study is a clear advance in the quest for an artificial pancreas that can be used by a diabetic while performing normal daily activity.
However, as the authors admit, one limitation is the exclusivelymanual control of the artificial pancreas used relied on study personnel to transmit data manually from the continuous glucose monitor (CGM) to the computer running the closed loop control, and to transmit insulin injection recommendations from the computer to the insulin pump because of technological and regulatory barriers
In fully automated systems ‒ which is what researchers and medical device makers are hoping to make a reality for diabetics ‒ these processes are handled by data transmission and pump control devices, respectively. However, Cambridge method limited the investigation to testing only the control algorithm, not the artificial pancreas as a whole. The testing of other key components, such as sensor-pump communication and error mitigation, would require much more effort and thorough system validation.
Studies using fully automated systems have already been reported by the Artificial Pancreas Project and offer hope for the future of ambulatory systems i.e. devices that be worn by diabetics on their person in their daily lives.
Lastly, despite the sophistication of the control algorithm and the significant reduction in nocturnal hypoglycemia, four episodes of severe hypoglycemia (<70 mg/dl) occurred, three of which the authors thought were attributable to the preceding prandial insulin dose and could not be prevented by the artificial pancreas suspending insulin delivery.
This finding reinforces the recently proposed idea that a dedicated hypoglycemia safety system ‒ a separate algorithm responsible solely for the assessment and mitigation of the risk of hypoglycemia ‒ may need to accompany closed loop control. Such safety systems already exist, and have proved useful.
Based on ‘Boris Kovatchev: Closed Loop Control For Type 1 Diabetes (BMJ 2011; 342:d1911)
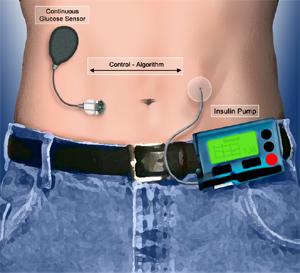 |
| Artificial pancreas concept |
The newer systems link a continuous glucose monitor and a subcutaneous insulin infusion pump via a control algorithm, which retrieves continuous glucose monitoring data in real time (for example, every five minutes) and uses a mathematical formula to compute insulin delivery rates that are then transmitted to the insulin pump.
However, artificial pancreas that can be worn by diabetics on their person as they go about their daily lives is still in development, with the first in-clinic studies now being reported. Preliminary results have been promising ‒ the most notable improvement is in overnight control of type 1 diabetes, with improvements in safety and a reduction in nocturnal hypoglycemia being reported.
These improvements result from the fine adjustment of insulin delivery provided by closed loop control overnight being superior to a generally fixed basal rate and less likely to cause hypoglycemia. The first application of closed loop control is therefore likely to be in glucose regulation overnight, a step that has the potential to improve dramatically the safety of insulin delivery during crucial, generally unsupervised, periods.
Now a University of Cambridge research tem led by Roman Hovorka has demonstrated the safety and efficacy of overnight closed loop insulin delivery with conventional insulin pump therapy in adults with type 1 diabetes.
The trial group consisted of 24 adults (10 men and 14 women) aged 18-65, who had used insulin pump therapy for at least three months and the research team used two protocols ‒ a medium sized meal (60 g carbohydrate) and a large size meal (100 g carbohydrate + alcohol) ‒ to see whether artificial pancreas were effective in overcoming nocturnal hypoglycemia.
As in previous studies carried out by Boris Kovatchev and others in the U.S. and France, the Cambridge closed loop system significantly increased the time that plasma glucose was in the target range (70-144 mg/dl), reduced incidence of hypoglycemia, and better overnight control.
But what makes the Cambridge study important is that the randomized crossover trial design is virtually unique in the field of closed loop control. Because this design is the gold standard for clinical research, the results set a benchmark for future studies.
The only other randomized controlled trial of closed loop control was recently presented by the University of Virginia research team led by Kovatchev at the 4th International Conference on Advanced Technologies and Treatments for Diabetes. This study recruited 24 adults and adolescents with type 1 diabetes in the United States and in France and achieved results similar to those reported by Hovorka and colleagues ‒ more time within the target range of 70-180 mg/dl and a threefold reduction in hypoglycemia.
However, artificial pancreas that can be worn by diabetics on their person as they go about their daily lives is still in development, with the first in-clinic studies now being reported. Preliminary results have been promising ‒ the most notable improvement is in overnight control of type 1 diabetes, with improvements in safety and a reduction in nocturnal hypoglycemia being reported.
These improvements result from the fine adjustment of insulin delivery provided by closed loop control overnight being superior to a generally fixed basal rate and less likely to cause hypoglycemia. The first application of closed loop control is therefore likely to be in glucose regulation overnight, a step that has the potential to improve dramatically the safety of insulin delivery during crucial, generally unsupervised, periods.
Now a University of Cambridge research tem led by Roman Hovorka has demonstrated the safety and efficacy of overnight closed loop insulin delivery with conventional insulin pump therapy in adults with type 1 diabetes.
The trial group consisted of 24 adults (10 men and 14 women) aged 18-65, who had used insulin pump therapy for at least three months and the research team used two protocols ‒ a medium sized meal (60 g carbohydrate) and a large size meal (100 g carbohydrate + alcohol) ‒ to see whether artificial pancreas were effective in overcoming nocturnal hypoglycemia.
As in previous studies carried out by Boris Kovatchev and others in the U.S. and France, the Cambridge closed loop system significantly increased the time that plasma glucose was in the target range (70-144 mg/dl), reduced incidence of hypoglycemia, and better overnight control.
But what makes the Cambridge study important is that the randomized crossover trial design is virtually unique in the field of closed loop control. Because this design is the gold standard for clinical research, the results set a benchmark for future studies.
The only other randomized controlled trial of closed loop control was recently presented by the University of Virginia research team led by Kovatchev at the 4th International Conference on Advanced Technologies and Treatments for Diabetes. This study recruited 24 adults and adolescents with type 1 diabetes in the United States and in France and achieved results similar to those reported by Hovorka and colleagues ‒ more time within the target range of 70-180 mg/dl and a threefold reduction in hypoglycemia.
 |
| Dr Roman Hovorka |
Moreover, the control algorithm used by Hovorka and colleagues belongs to an advanced class of closed loop control technologies known as “model predictive control”. Algorithm designs for artificial pancreas have generally used either “proportional-integral-derivative control” or “model predictive control”.
Proportional-integral-derivative control algorithms are reactive, responding to changes in glucose levels with adjustment in insulin delivery. Model predictive control algorithms are built over a model of the human metabolic system and are therefore proactive, delivering insulin in anticipation of changes in glucose concentrations.
This compensates partially for the time delays inherent in subcutaneous glucose control (the time delay in insulin action, which can amount to 60 minutes or more). For this reason, model predictive control has become the approach of choice more recently.
The algorithm developed by Hovorka and colleagues has certain distinct features, such as real time adaptation of the underlying model to changing patient parameters implemented as a selection from several predefined models. However, this potential advantage remains to be evaluated.
Most importantly, this is one of the first studies to test realistic meal scenarios and challenge the participants with a large dinner that included alcohol. As such, the study is a clear advance in the quest for an artificial pancreas that can be used by a diabetic while performing normal daily activity.
However, as the authors admit, one limitation is the exclusivelymanual control of the artificial pancreas used relied on study personnel to transmit data manually from the continuous glucose monitor (CGM) to the computer running the closed loop control, and to transmit insulin injection recommendations from the computer to the insulin pump because of technological and regulatory barriers
In fully automated systems ‒ which is what researchers and medical device makers are hoping to make a reality for diabetics ‒ these processes are handled by data transmission and pump control devices, respectively. However, Cambridge method limited the investigation to testing only the control algorithm, not the artificial pancreas as a whole. The testing of other key components, such as sensor-pump communication and error mitigation, would require much more effort and thorough system validation.
Studies using fully automated systems have already been reported by the Artificial Pancreas Project and offer hope for the future of ambulatory systems i.e. devices that be worn by diabetics on their person in their daily lives.
Lastly, despite the sophistication of the control algorithm and the significant reduction in nocturnal hypoglycemia, four episodes of severe hypoglycemia (<70 mg/dl) occurred, three of which the authors thought were attributable to the preceding prandial insulin dose and could not be prevented by the artificial pancreas suspending insulin delivery.
This finding reinforces the recently proposed idea that a dedicated hypoglycemia safety system ‒ a separate algorithm responsible solely for the assessment and mitigation of the risk of hypoglycemia ‒ may need to accompany closed loop control. Such safety systems already exist, and have proved useful.
Based on ‘Boris Kovatchev: Closed Loop Control For Type 1 Diabetes (BMJ 2011; 342:d1911)
Related Post: Killer Apps That Are Revolutionizing Diabetes Care







